WHAT IS THE OXIDATION
View all Supplements Antioxidants
DEFINITION OF OXIDATION:
In chemistry, it is said that an element undergoes oxidation when it undergoes a subtraction of electrons, which results in the increase of its oxidation number. Each oxidation occurs simultaneously with a reduction in a process that takes the generic name of oxidation-reduction, often abbreviated as redox
.
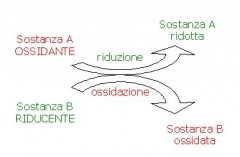
A reaction of oxidation-reduction (redox reaction) is a reaction in which a substance loses electrons:
then the substance losing electrons (oxidizes), while another substance buys them (is reduced).
The chemical species which oxidizes yields electrons increases and the number of oxidation (> no).
The chemical species that is reduced buy those electrons, decreasing the number of oxidation,
Obviously the chemical species which oxidizes acts as a reductant while, on the contrary, one that reduces acts as oxidant.
Oxidize means therefore have a more positive charge (+) first and Shrink means having a more negative charge (-) before.
Oxidation and reduction always occur simultaneously, for the transfer of electrons from one atom to another.
In a reaction oxido-reductive balance of charges must be equal to zero.
OXIDATION IN OUR BODY:
The cellular metabolism and the eventual activation of reactive processes (such as, for example, inflammation) or detoxification, give the circulating blood the ability to oxidize, ie to tear electrons or hydrogen atoms to certain chemical species.
The so-called oxidative stress arises when the balance between oxidation and reduction reactions in the body is unbalanced in favor of processes which promote oxidation.
MEASURING THE OXIDATION:
The d-ROMs test, the only test recommended by the international scientific community and the International Observatory of Oxidative Stress, is the only test currently available for the assessment of oxidative stress.
The d-ROMs test measures the ability "oxidant" of a plasma sample with regard to a particular substance (an aromatic amine modified) used as an indicator (chromogen). The phenomenon is associated with the gradual and progressive color change towards the pink of the reaction mixture (plasma + chromogen), initially colorless. The color change measured by a particular device (photometer) that, ultimately, converts it into a "number" the oxidizing capacity thus determined.
View all Supplements Antioxidants
